Scientists have created a once-theoretical molecule under space-like conditions, revealing new insights into the chemistry of the cosmos and the origins of complex compounds.
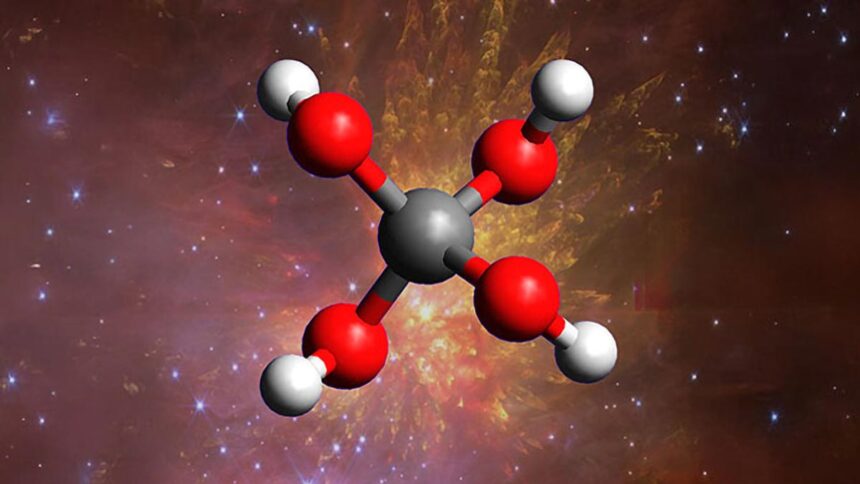
Scientists from the University of Hawaiʻi at Mānoa’s Department of Chemistry have successfully synthesized methanetetrol, a molecule long believed too unstable to naturally exist. By replicating extreme conditions similar to those found in deep space, their breakthrough offers new insight into how complex chemical reactions may occur in the cosmos.
Methanetetrol stands out as the only known alcohol with four hydroxyl groups (OH) bonded to a single carbon atom. Although its theoretical existence has been proposed for over 100 years, this marks the first time it has ever been observed. The team achieved this by mimicking the frigid, near-vacuum environment of interstellar clouds and exposing materials to intense radiation, creating the conditions necessary for methanetetrol to form.
Complex compounds, building blocks of life
This finding shows that outer space may host a far more diverse and unexpected set of chemical reactions than previously believed. These reactions are critical to understanding the formation of organic molecules (building blocks of life) across the galaxy. By proving that methanetetrol can form under cosmic conditions, the team has revealed a surprising pathway for how complex compounds might evolve in the icy dust clouds where stars and planets form.
The team used powerful vacuum ultraviolet light to detect tiny amounts of methanetetrol made from water and carbon dioxide. They found that high-energy particles mimicking high-energy cosmic rays triggered a series of chemical reactions leading to the creation of methanetetrol and related compounds.
“In collaborations with scientists from Mississippi, Samara University, and Shanghai, this work pushes the boundaries of what we know about chemistry in space,” said Department of Chemistry Professor Ralf I. Kaiser.
While this alcohol does not occur naturally on Earth due to its instability in everyday conditions, its formation in space demonstrates that the universe is far more chemically dynamic than previously imagined. The findings push the boundaries of both chemistry and astronomy, and open the door to further discoveries and astronomical observations about how life’s ingredients can emerge in the coldest, darkest corners of space.