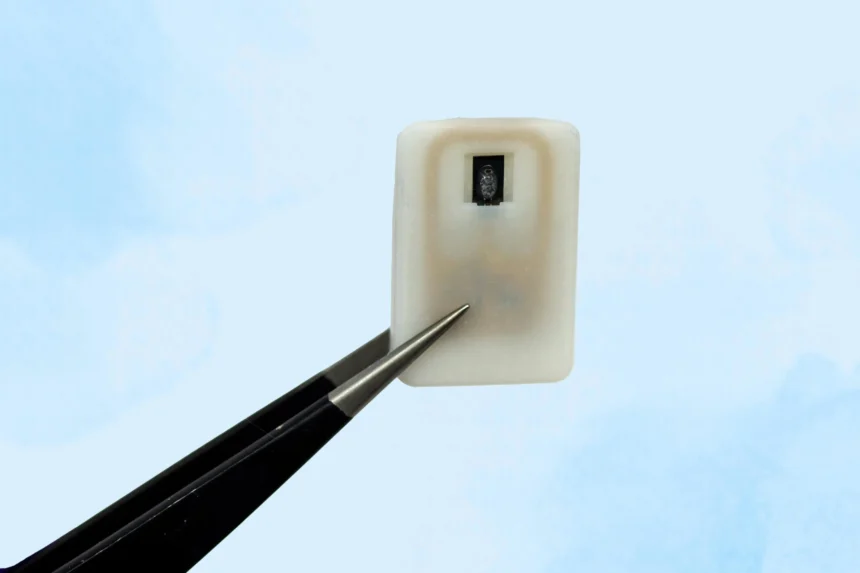
The new implant contains a reservoir of glucagon that sits beneath the skin and can be activated in an emergency, with no need for injections.
For individuals with Type 1 diabetes, the risk of hypoglycemia, dangerously low blood sugar, is a constant concern. When glucose levels drop too far, the condition becomes life-threatening and typically requires an injection of the hormone glucagon as the standard treatment.
To address situations where patients may be unaware that their blood sugar is falling to critical levels, MIT engineers have developed an implantable device that holds a supply of glucagon beneath the skin. This device can be activated to release the hormone automatically when glucose drops too low.
The system could be especially useful during nighttime hypoglycemia or for young children with diabetes who may not be able to self-administer an injection.
“This is a small, emergency-event device that can be placed under the skin, where it is ready to act if the patient’s blood sugar drops too low,” says Daniel Anderson, a professor in MIT’s Department of Chemical Engineering, and a member of both the Koch Institute for Integrative Cancer Research and the Institute for Medical Engineering and Science (IMES). “Our goal was to build a device that is always ready to protect patients from low blood sugar. We think this can also help relieve the fear of hypoglycemia that many patients, and their parents, suffer from.”

The researchers also demonstrated that the same technology could be adapted to deliver emergency doses of epinephrine, a medication used to treat heart attacks and prevent severe allergic reactions such as anaphylaxis.
The study was led by Siddharth Krishnan, a former MIT research scientist and now an assistant professor of electrical engineering at Stanford University. The findings were published on July 9, 2025, in Nature Biomedical Engineering.
Emergency response
Many people with type 1 diabetes rely on daily insulin injections to help regulate their blood sugar and prevent it from rising too high. However, when blood sugar drops too low, it can lead to hypoglycemia—a condition that may cause disorientation, seizures, and in severe cases, death if not promptly treated.
To address this, some individuals carry prefilled syringes of glucagon, a hormone that signals the liver to release stored glucose into the bloodstream. Still, recognizing the early signs of hypoglycemia can be challenging, particularly for children.
“Some patients can sense when they’re getting low blood sugar, and go eat something or give themselves glucagon,” Anderson says. “But some are unaware that they’re hypoglycemic, and they can just slip into confusion and coma. This is also a problem when patients sleep, as they are reliant on glucose sensor alarms to wake them when sugar drops dangerously low.”
To provide a more reliable solution, the MIT team developed a compact emergency device that can be activated manually or automatically in response to low blood sugar, based on sensor input.
Roughly the size of a quarter, the device houses a small drug reservoir fabricated using 3D-printed polymer. This reservoir is sealed with a material called a shape-memory alloy, engineered to change form when exposed to heat. The team used a nickel-titanium alloy that transitions from a flat slab to a U shape when heated to 40 degrees Celsius.
Since glucagon, like many peptide-based drugs, degrades quickly in liquid form, the researchers opted for a powdered formulation that remains stable over extended periods and stays inside the device until needed.
Each unit can store one or four doses of glucagon and contains an antenna that responds to a specific radiofrequency signal. When triggered, the antenna activates a small electrical current that heats the shape-memory alloy. Once the material reaches the activation temperature, it bends into a U shape and releases the powdered drug from the reservoir.
Because the device can receive wireless signals, it could also be designed so that drug release is triggered by a glucose monitor when the wearer’s blood sugar drops below a certain level.
“One of the key features of this type of digital drug delivery system is that you can have it talk to sensors,” Krishnan says. “In this case, the continuous glucose-monitoring technology that a lot of patients use is something that would be easy for these types of devices to interface with.”
Reversing hypoglycemia
After implanting the device in diabetic mice, the researchers used it to trigger glucagon release as the animals’ blood sugar levels were dropping. Within less than 10 minutes of activating the drug release, blood sugar levels began to level off, allowing them to remain within the normal range and avert hypoglycemia.
The researchers also tested the device with a powdered version of epinephrine. They found that within 10 minutes of drug release, epinephrine levels in the bloodstream became elevated and heart rate increased.
In this study, the researchers kept the devices implanted for up to four weeks, but they now plan to see if they can extend that time up to at least a year.
“The idea is you would have enough doses that can provide this therapeutic rescue event over a significant period of time. We don’t know exactly what that is — maybe a year, maybe a few years, and we’re currently working on establishing what the optimal lifetime is. But then after that, it would need to be replaced,” Krishnan says.
Typically, when a medical device is implanted in the body, scar tissue develops around the device, which can interfere with its function. However, in this study, the researchers showed that even after fibrotic tissue formed around the implant, they were able to successfully trigger the drug release.
The researchers are now planning for additional animal studies and hope to begin testing the device in clinical trials within the next three years.
“It’s really exciting to see our team accomplish this, which I hope will someday help diabetic patients and could more broadly provide a new paradigm for delivering any emergency medicine,” says Robert Langer, the David H. Koch Institute Professor at MIT and an author of the paper.